Gypsum
Benefits and Misconceptions
Each year, usually in the winter and early spring, we receive calls asking about the benefits of applying gypsum to Midwestern or Eastern farm fields. Typically, the grower is looking for a way to reduce soil compaction. This paper is intended to answer many of these questions.
Gypsum is a naturally occurring mineral in many parts of the United States and the world. Gypsum is also often available as a by-product material. Gypsum is hydrated calcium sulfate. The chemical name of gypsum is calcium sulfate dihydrate. The chemical formula for pure gypsum is Ca(SO4)•2(H2O). In the chemically pure form, gypsum contains 23.28% calcium (Ca) and 18.62% sulfur (S) in the readily available sulfate form (SO4). However, the typical gypsum sources that are commercially available for agricultural often contain impurities which result in a Ca level between 18%-23% Ca and sulfur levels between 15%-19%. Gypsum is also available as a byproduct material. Some of the more common byproduct gypsum sources are flue gas desulfurization (FGD) gypsum and waste wallboard (also known as sheet rock or drywall). A few locations may have access to byproduct phosphogypsum from the production of P fertilizer, and some locations may have access to byproduct gypsum sources related to other industrial processes.
Gypsum has many uses in addition to agriculture. A partial list of products and processes that use gypsum includes blackboard chalk, cement, wallboard, Plaster-of-Paris, dental molds, paint filler, toothpaste, molds for casting metals, Tofu coagulation, improving mineral content of brewing water, dietary calcium additives in breads and cereals, and pharmaceuticals.
| Table 1. Mineralogical composition of gypsum samples | |
|---|---|
| Source | Mineralsa Present |
| Synthetic gypsumb | gypsum, quartz |
| Natural gypsumc | gypsum, quartz, dolomite |
| Cast gypsumd | gypsum, quartz, anhydrite |
| Drywall gypsume | gypsum, quartz, portlandite, calcite |
| a gypsum = CaSO4•2H2O, quartz = SiO2, dolomite = CaMg(CO3)2, anhydrite = CaSO4, portlandite = Ca(OH)2, calcite = CaCO3 | |
| b Flue gas desulfurization (FGD) by-product. Samples obtained from the W.H. Zimmer Station in Moscow, OH, owned by Cinergy Corporation | |
| c Mined geologic deposits. Samples obtained from the Kwest Group at Port Clinton, OH | |
| d Cast gypsum from recycled molds. Samples obtained from Mansfield Plumbing Products, LLC of Mansfield, OH | |
| e Waste wallboard/drywall/sheet rock. Samples obtained from Transfer Services, LLC of Columbus, OH | |
Ohio State University Extension Fact Sheet (ANR-20-05) entitled “Gypsum for Agricultural Use in Ohio-Sources and Quality of Available Products” lists some sources and properties of gypsum available in Ohio. This information is probably typical of that found in many places and is presented as an example of material that may be in your area. Please note that the descriptions in table 1 have been edited by Spectrum Analytic, Inc to clarify the description of the materials.
| Table 2. Physical properties and price (as of 12/2004) of gypsum | ||||
|---|---|---|---|---|
| Material | Water contenta % | Particle size | Price $/ton | Insoluble residueb % |
| Synthetic gypsum | 5.55 (3.04)c | 120 µm | 7.00 | 0.4 (0.2) |
| Natural gypsum | 0.38 (0.48) | NAd | 12.75 | 12.9 (8.1) |
| Cast gypsume | 0.15 (0.21) | NA | NA | 0.2 |
| Drywall gypsum | 10.1 (12.8) | < 0.5 inch | 11.00 | 2.2 (0.3) |
| a Dried overnight at 60 degrees Celsius. | ||||
| b Following dissolution for three days at pH < 3. | ||||
| c Standard deviation included in parentheses. | ||||
| d NA = not available | ||||
| e Material is not yet available for sale for agricultural application. | ||||
| Table 3. Selected macro- and micronutrienta concentrations in the gypsum samples | |||||||
|---|---|---|---|---|---|---|---|
| Measure | Units | Museum specimenb | Synthetic gypsum | Natural gypsum | Cast gypsum | Drywall gypsum | Ideal analysisc |
| Calcium | % | 22.6 | 23.0 (0.0)d | 19.1 (2.2) | 22.4 (0.0) | 21.9 (0.2) | 23.3 |
| Magnesium | % | 0.01 | 0.03 (0.01) | 1.35 (0.30) | 0.05 (0.00) | 0.22 (0.01) | |
| Sulfur | % | 18.6 | 18.7 (0.1) | 15.1 (1.2) | 19.3 (0.2) | 18.1 (0.3) | 18.6 |
| Boron | ppm | < 13.1 | 26.7 (8.7) | 9.4 (0.9) | 0.4 (0.4) | 7.3 (4.5) | |
| Iron | ppm | < 1 | 264 (129) | 1045 (148) | 44 (7) | 547 (92) | |
| Manganese | ppm | 0.1 | 5.5 (2.3) | 14.6 (2.9) | 9.1 (0.0) | 9.4 (1.6) | |
| Phosphorus | ppm | 3.8 | 16.7 (9.4) | 30.6 (7.6) | 7.5 (0.3) | 51.6 (3.5) | |
| a Micronutrient data obtained by EPA method 3050 (USEPA, 1996). | |||||||
| b The museum specimen is included as a pure sample of gypsum. | |||||||
| c Calculated content in a 100% pure product. | |||||||
| d Standard deviation included in parentheses. | |||||||
| Table 4. Trace metal contenta of gypsum from different sources compared with U.S. EPA Part 503 pollutant concentration limits for excellent quality biosolids. | ||||||
|---|---|---|---|---|---|---|
| Pollutant (ppm = mg kg-1) | Museum specimen | Synthetic gypsum | Natural gypsum | Cast gypsum | Drywall gypsum | Part 503 Table 3b |
| Arsenic | < 0.52 | 0.56 (0.05)c | < 0.52 | < 0.52 | 0.98 (0.11) | 41 |
| Cadmium | < 0.48 | < 0.48 | < 0.48 | < 0.48 | < 0.48 | 39 |
| Chromium | 0.01 | 1.30 (0.85) | 1.38 (0.32) | 0.07 (0.00) | 1.09 (0.09) | 1200 |
| Cobalt | < 0.48 | < 0.48 | 0.53 (0.04) | < 0.48 | < 0.48 | NRd |
| Copper | < 0.48 | 1.16 (0.66) | 1.33 (0.30) | 1.40 (0.21) | 0.95 (0.14) | 1500 |
| Lead | < 0.48 | 0.80 (.30) | 2.92 (0.30) | 0.57 (0.08) | 0.70 (0.02) | 300 |
| Mercury | < 0.26 | < 0.26 | < 0.26 | < 0.26 | < 0.26 | 17 |
| Molybdenum | < 0.24 | 0.51 (0.26) | 1.28 (0.04) | < 0.24 | < 0.24 | -e |
| Nickel | < 0.24 | 0.73 (0.18) | 1.42 (0.23) | < 0.24 | 0.83 (0.12) | 420 |
| Selenium | < 1.45 | 5.51 (3.47) | < 1.45 | < 1.45 | 1.85 (0.04) | 36 |
| Zinc | < 0.24 | 3.88 (2.78) | 0.91 (0.49) | < 0.24 | 3.08 (0.45) | 2800 |
| a Data obtained by EPA method 3050 (USEPA, 1996). | ||||||
| b Part 503-Standards for the Use or Disposal of Sewage Sludge; 503.13, Table 3. (USEPA, 1993). | ||||||
| c Standard deviation included in parentheses. | ||||||
| d NR = not regulated. | ||||||
| e Ceiling concentration limit for molybdenum is 75 ppm; 503.13, Table 1. (USEPA, 1993). | ||||||
"Claimed" Uses for Gypsum
As a soil amendment, gypsum has the following proven benefits.
- Correcting the damaging effects of high soil sodium (Na)
- A source of readily plant-available Ca
- A source of readily plant-available SO4-S
- Increase the pH of highly acid subsoils
- Reducing Al toxicity of highly acid subsoil
- Possible benefits by lessening the severity of soil surface crusting
- Gypsum can reduce ammonia volatilization from urea and UAN fertilizers
These are the only demonstrated benefits derived from applying gypsum.
Misconceptions about Gypsum
There is a lot of confusion and misrepresentation about the benefits of gypsum for soils and plants. The following list is in response to the most common misconceptions.
- Gypsum will not correct the most common type of soil compaction
- High quality gypsum will not change the soil pH, although contaminants in some by-product gypsum may have some effect (raising or lowering pH) on soils.
- Gypsum will not “de-toxify” most soil problems.
Gypsum Effects on Soil Compaction
Typically, there are three types of soil compaction that prompt growers to ask about using gypsum. They are…
1. Relatively deep and thick layers of compacted soil caused by mechanical compaction of clay-based soils
2. Surface crusting
3. Loss of soil structure caused by high sodium (Na) levels in the soil
1. One of the most common misconceptions is that gypsum will help reduce mechanical soil compaction in clay soils that are not damaged by excess Na (sodic soils). There is no research data to support the claim that gypsum has any significant effect on reducing mechanical soil compaction. The belief that gypsum will reduce soil compaction probably comes from its effect on crusting and sodic soils. It needs to be understood that both soil crusting is caused by a different process than mechanical compaction and that compaction caused by sodic soils is extremely rare in the vast majority of fields in this country. Nearly all compaction affecting farms is caused by production practices that mechanically compact the soil, not by a chemical process. Mechanical compaction is caused by field operations performed when the land was too wet and/or improper implement selection. It can only be corrected through proper soil management practices, such as deep tillage, not working the field when it is too wet, proper equipment selection, and others.
| Table 5. Application of Flue-Gas Scrubber Desulfurization Sludge (Gypsum) | |||||||
|---|---|---|---|---|---|---|---|
| Product Rate | 3-yr Corn Yield | 4-yr Soybean Yield | Soil pH 3-yr | Exchangeable 1-yr | 1 yr Bulk Density | ||
| Ca | Mg | ||||||
| lb/a | bu/a | bu/a | ppm | ppm | g/cu. cm. | ||
| 0 | 159 | 36 | 6.8 | 1608 | 407 | 1.42 | |
| 1,000 | 159 | 38 | 6.9 | 1615 | 371 | 1.41 | |
| 10,000 | 156 | 36 | 6.8 | 1705 | 368 | 1.45 | |
| 50,000 | 142 | 35 | 6.7 | 2110 | 330 | 1.38 | |
| 100,000 | 145 | 33 | 6.9 | 3960 | 294 | 1.39 | |
| Significant | S | S | NS | S | S | NS | |
| Wynoose silt loam (F. Thicke, Ph.D. Thesis, 1988, Univ. Illinois) Material applied spring 1984, moldboard plow incorporation. Newton, IL |
|||||||
2. Soil crusting forms when the clay in the surface layer of soil becomes dispersed in water, then settles to form a thin, layer of interlocking clay particles on the soil surface. As this layer of deposited clay dries, it hardens and forms a barrier between the soil and the air. Such a crust can significantly reduce the water infiltration rate as well as the exchange of gasses with the atmosphere. This type of soil crust can increase erosion as well as degrade the growing conditions for the crop. As the thickness and strength of the crust increases, it may reach a point at which it physically inhibits seedling emergence. Several researchers have found that increasing the Ca saturation of the surface layer of the soil can reduce the possibility of crust formation as well as the damage caused by crust formation. The benefit of increasing the Ca saturation of the surface soil appears to be more important in soils with a high Mg content. Finely ground gypsum can be effective, at least in the short term, in reducing soil crusting in such soils when applied in the appropriate manner. It is recommended that gypsum applied to prevent or reduce soil crusting be surface applied and not incorporated. North Carolina State University recommends rates between 500 to 2,000 lb. of gypsum per acre for this purpose. Remember, soil crusting is not the same thing as mechanical soil compaction. Different processes are at work in the two problems and the fact that gypsum helps to alleviate crusting does not mean that it will improve compacted soils. Also, we found no data that compared the relative effectiveness of gypsum with mechanical cultivation to break soil crust. The effect of raindrop impact on bare soil is one of the factors that increase soil crusting in susceptible soil types. Therefore, the proper management of crop residues to shield the soil from raindrop impact is recommended as part of the soil management system.
3. Gypsum has long been recognized as being effective at improving the soil structure in sodic soils. When soil contains enough excess Na, the soil is unable to form aggregates. The result is similar to crusting, only it involves the entire topsoil layer, not just a fraction of an inch on the surface. Very few fields east of the dryer parts of the Great Plains have sodic soils that might benefit from gypsum. We find some in small areas around old oil wells where large volumes of salt water were pumped out onto the surface of the soil. Some years ago, researchers found that if they applied large amounts of gypsum (1-10 tons/acre) followed by large amounts of irrigation water, the excess Ca from the gypsum displaces the Na in the soil and the excess water leaches the displaced Na below the root zone of the planned crop. This permits a crop to be grown successfully on that land. Of course, the excess Ca will also displace other nutrient cations such as K and Mg, so the fertility program would need to be adjusted to compensate for this loss of nutrients. From this you can see that if a soil does not have excess Na there is no mechanism for gypsum to provide relief from this source of compaction.
Gypsum Effects On Soil pH
This topic can get a little confusing due to the chemistry and causes of soil acidity, and the different ways in which lime and gypsum react in the soil. The short answer is that pure gypsum will not affect the pH of the topsoil when surface applied or incorporated by typical methods. However, gypsum is able to offset some of the toxic effects of extremely acid subsoil, in some soil types. Gypsum may even increase the pH of those subsoils to some degree. These seemingly contradictory statements can be explained by the differences in the chemistry between lime and gypsum, plus the somewhat unique nature of most acid subsoils.
Some people think that gypsum will increase soil pH. This belief is apparently based on fact that gypsum contains a significant amount of Ca. Without getting too deeply into the chemistry of lime, a short description of how lime works will help to illustrate why gypsum will not neutralize soil acid.
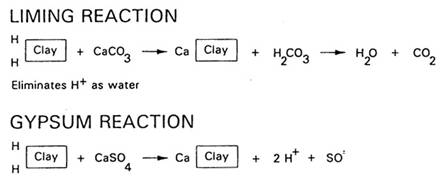
Typical agricultural lime is primarily composed of calcium carbonate (CaCO3) and magnesium carbonate (MgCO3). The principle acid-neutralizing power of agricultural lime is caused by the carbonate (CO3) in both the calcium carbonate (CaCO3) and magnesium carbonate (MgCO3), not so much by the Ca nor Mg. Both lime minerals work in the same way to neutralize acid. Soil acid is defined as the hydrogen ion (H+) content of the soil. To neutralize this acid, any soil amendment must either convert the H+ into another non-acidic form of H or cause the minerals which generate the H+ to stop or slow that process. Carbonates act directly upon the H+ by causing it to combine with carbon dioxide (CO2) to form water (see illustration). Since gypsum does not change the form of soil H+ there is no change in soil pH.
The beneficial effect of gypsum on some acid subsoil occurs by a different type of chemistry. If acid neutralizing power were the only consideration, then lime would be the material of choice to neutralize acid subsoils. The problem with lime is that it is not very mobile in the soil. Therefore, in order to neutralize acid subsoils in any reasonable amount of time, the lime must be physically mixed into the subsoil. This is not something that most farmers can accomplish. Gypsum can affect subsoil acidity in a shorter time frame because it is much more mobile in the soil and can be leached into the subsoil by irrigation or rainfall.
In extremely acid subsoils (pH 5.0 and lower) excess soluble aluminum (Al+++) is the main problem. Excess soluble Al is toxic to plants. The primary damage from excess Al+++ is the death of the root growing points or root pruning. The non-toxic calcium ion (Ca++) generated by gypsum is a competitive with Al+++. When the Ca++ from the gypsum reaches the subsoil, it causes some of the Al+++ to be leached into greater soil depths (assuming enough water passes through the subsoil). The more Ca++ that leaches through the subsoil, the more Al+++ is leached. The same benefit would occur in the topsoil if it were at the same pH. However, in this case, we can use lime in which we get the benefits of acid neutralization plus the effects of Ca and Mg.
Neither the lime nor the gypsum is an instant solution to excess Al+++. Depending on the nature and particle size of lime, it could require up to 18 months for the lime to completely react and neutralize acid topsoil. Gypsums effects on subsoil can take less time, depending on how fast it can be leached through the subsoil.
Gypsum as a Nutrient Source
Because gypsum is highly soluble, it is an excellent source of Ca and S, especially for acid-loving crops and ornamentals, plus a few crops that are especially responsive to either Ca or S for reasons other than soil pH. Acid soils are by nature low in Ca. Where additional Ca is needed, gypsum is an ideal source for these crops. Potatoes are often grown in acid soil to control common scab. In these conditions, gypsum can improve tuber quality. Commercial Christmas tree producers have greatly improved the quality of their acid-loving species with applications of gypsum. Blueberry producers have also found gypsum to be benefit with that acid-requiring crop. The list of acid-loving ornamentals is much too long to include here, but any of these plants are likely to benefit from additional Ca that does not increase the soil pH. While peanuts do not require acid soils, they are very responsive to applied Ca as gypsum.
Calculating Gypsum Requirement to Reduce soil Na and to build Soil Ca.
These two uses for gypsum require somewhat more complicated calculations in order to determine the appropriate rate of application. Reducing soil Na levels is by far the more complicated process and the following is only a superficial discussion of correcting this problem. A much more in-depth discussion is presented in Spectrum Analytics paper entitled “A Guide to Interpreting Irrigation Water Analysis”, which can be found in the Library at spectrumanalytic.com.
Reducing Soil Sodium
- Reducing Na to a “generally acceptable” level:
The following formula is published by North Carolina State University.
- Reducing Na to a particular saturation percent:
The following calculation may be used when there is a specific percent Na saturation target to be achieved.
Example
Soil CEC = 20; Na % saturation = 40%; Na % saturation goal = 10% Gypsum = 80% efficient at displacing excess soil Na
Calculations
(Present % Na - Goal % Na ) = 30%
30% of 20 CEC, which is 0.30 × 20 = 6 meq. of Na
6 meq. of Na ( 0.85 Tons of gypsum per meq. of Na = 5.1 Tons gypsum/ac (at 100% efficiency)
5.1 Tons/a at 80% efficiency, which is 5.1 Tons/ac / 0.80 = 6.38 Tons gypsum/acre final requirement
- Calculating gypsum to offset Na in irrigation water
Gypsum requirements can be calculated from the residual sodium carbonate (RSC) 1) value of the irrigation water from the following equation. RSC × 234 = pounds of gypsum required to offset the excess sodium in 1 acre foot (325,852 gallons) of irrigation water
Remember, gypsum alone does not solve a high Na problem, you must apply adequate irrigation water (or wait for enough rainfall) to leach the displaced Na out of the root zone.
Increasing Soil Ca Saturation
This calculation is to simply increase the soil Ca to some desired percent Ca saturation through the use of gypsum. The formula was published by North Carolina State University.
The Myth of an Ideal Calcium to Magnesium Ratio
Gypsum is sometimes recommended in order to adjust the soil Ca:Mg ratio to some desired value. The previous formula designed by N.C. State Univ. to Increase the soil Ca saturation could be used to achieve this goal. However, The idea that there is some “ideal” soil Ca:Mg ratio is a myth, and not worth pursuing. Competition between Ca and Mg for uptake by crops has become a perennial topic of discussion in agriculture. Generally, the discussion centers on the claim by some that there is an “ideal” soil Ca/Mg ratio that should be achieved through fertilization. Often times, the ideal Ca:Mg ratio is somewhere between 5:1 and 8:1. Some of the claimed benefits of this ideal soil Ca:Mg ratio include
- Improved soil structure.
- Reduced weed populations, especially foxtail and quackgrass, plus improved forage quality.
- Reduced leaching of other plant nutrients.
- Generally improved balance of most soil nutrients.
The first publication of an ideal Ca/Mg ratio probably came from New Jersey in 1901. This early work recommended a “total” Ca to “total” Mg ratio in the soil of about 5/4. As we know today, the “total” soil content of CA, Mg, or any nutrient has little relationship to its availability or uptake by crops. It also has literally nothing to do with the general fertility of the soil. In a 1945, a publication by Bear, again in New Jersey, suggested that an example of an ideal soil was one that had the following saturations of exchangeable cations 65% Ca, 10% Mg, 5% K, and 20% H. The cation ratios resulting from these idealizes concentrations are a Ca:Mg of 6.5:1, Ca:K of 13:1, and Mg:K of 2:1 (or a K:Mg or 0.5:1). It appears that since this time, these ratios have been seized upon by some people as the “ideal” ratios, rather than examples of good ratios.
Since these published figures, we have seen that many top yields of many different crops have been produced in soils with cation nutrient ratios much different than those first published. Also, a significant amount of research has looked at the question of nutrient ratios and almost no results have supported the claim of some “ideal” ratio. Fertile soils commonly have a Ca:Mg ratio between 5:1 and 8:1. However, this does not mean that the specific Ca:Mg ratio is required, best, or even related to yield. Research results show that this ratio can be as narrow as 2:1 or as wide as 11:1 without negative effects, assuming that there is an adequate amount of each nutrient in the soil.
In the mid-1980's the University of Wisconsin conducted research into the effect of Ca:Mg ratio on alfalfa growth. They found that while the Ca:Mg ratio in the plant tended to reflect the soil Ca:Mg ratio, the plant content of these nutrients was affected much less and in no case did the soil or plant ratio affect yield. In this work the plant Ca and Mg contents were never below the respective critical levels for each nutrient, even though the soil Ca:Mg ratios ranged from 2.28:1 to 8.44:1. They concluded that, assuming there are adequate levels of Ca and Mg present in the soil, variations in the Ca:Mg ratio over the range of 2:1 up to 8:1 have no effect on yield.
In 1999 the University of Missouri, Delta Research Center published the results of an investigation into the effects of soil Ca:Mg ratio on cotton. They amended plots with gypsum or Epsom salts to create soil Ca:Mg ratios between 3.8:1 and 11.7:1. They found that cotton yields were not significantly different between treatments.
McLean, et al in Ohio, could find no specific cation ratios that predicted sufficiency or shortages of K, Mg, or Ca in several crops (Table 1). Notice that for all crops the Ca:Mg ratios of both the high and low yielding groups have essentially the same ranges. There is no trend or bias in the relationships between the Ca:Mg ratio and the relative yields of any crop. This indicates that the soil Ca:Mg ratio had little or no effect on yield and the researchers concluded the same.
| Table 1 | |||||
|---|---|---|---|---|---|
| Cation Ratio | Yield Group | Ranges of Soil Ca/Mg Ratio | |||
| Corn | Soybeans | Wheat | Alfalfa | ||
| Ca/Mg | High | 5.7 - 20.6 | 5.7 - 14.9 | 5.7 - 14.0 | 6.8 - 26.8 |
| Ca/Mg | Low | 5.4 - 18.8 | 2.3 - 16.1 | 6.8 - 21.5 | 5.7 - 21.5 |
The obvious conclusion is that crop yields are not significantly affected by the soil Ca/Mg ratio as long as both nutrients are present in adequate amounts.
According to Dr. Stanley Barber, the noted soil scientist at Purdue University, “There is no research justification for the added expense of obtaining a definite Ca:Mg ratio in the soil. Research indicates that plant yield or quality is not appreciably affected over a wide range of Ca:Mg ratios in the soil.”
The Role of Gypsum in "De-Toxifying" Soils
Gypsum is sometimes recommended based on the ability of soluble Ca to counteract the effects of toxic levels of micronutrients and heavy metals in the soil. There is a significant amount of evidence that soluble Ca can in fact be beneficial in counteracting these problems. However, the practical and economic benefits of this chemistry to most growers are doubtful for the following reasons.
- Land with toxic levels of these metals, are extremely rare. Where the land is highly polluted, much more drastic remedies are required.
- Research on problems shows that where gypsum provides some benefit, it is relatively small.
- Nearly all toxicities found in farm soils or landscapes are related to extremely acid soils. Lime is the proper solution to those problems.
Some of the more common micronutrient toxicity questions are addressed in the following sections.
Boron Toxicity
Like the misunderstandings about gypsum's role in soil compaction, its role in alleviating B toxicity appears to be related to its use in reclaiming sodic soils. It is not uncommon for areas with high Na soils and irrigation water, to also have high levels of B in both the soil and water. In these areas, some or much of the B occurs in the form of easily soluble sodium metaborate. Applications of gypsum have been shown to convert much of the sodium metaborate to the much less soluble calcium metaborate. Where the dominant form of soil B is not sodium metaborate, which included most Midwestern and Eastern soils, the beneficial effects of gypsum for reducing excess soil B may be limited.
Another factor that may have increased the reputation of gypsum in alleviating B toxicity is the role of soil pH in reducing B availability. It is well known that a high soil pH (> 7.2) can greatly reduce the availability of soil B. Since the most common cause of high pH is excess applications or soil content of calcium carbonate (lime), the role of Ca might get confused with the separate role of pH. Having said this, it is commonly accepted that there is a relationship between Ca and B in both soils and plant physiology. Clemson University has reported that “The relationship between boron and several other nutrients has been established. Calcium, potassium, and nitrogen can affect boron nutrition. The calcium-boron relationship is the most important. Soils high in calcium will require more boron. Lower rates of boron will be required for soils low in calcium, and chances of boron toxicity are greater.” Based on this statement, if the chances of B toxicity are greater in low Ca soils, then it would seem logical that high soil Ca should reduce the chances of B toxicity. We were unable to find research results that proved the ability of gypsum to prevent or correct B toxicity to crops. It appears that in the event of an accidental application of excess B fertilizer, an emergency application of gypsum may offer a small chance of some benefit to the crop. However, it should not be expected to perform miracles.
Copper Toxicity
In preparing information for this paper, we ran across several claims of gypsum being instrumental in reducing the negative effects of excess soil Cu. After an extensive literature search on this subject, we found only one reference to this claim. In Reference 10 a list of recorded interactions of Cu with other elements in plant tissues listed the following reference… “Ca was shown to reduce Cu uptake in nutrient solution culture in lettuce”. A second reference to the effects of Ca on Cu uptake by a crop said… “Increasing Ca in solution culture improved reduced growth due to Cu toxicity in mungbean”. The most common recommendation for amending soils exhibiting Cu toxicity was to lime the soil to a pH of 6.5 or a little higher. For example, the University of Florida says “diagnosis of copper toxicity can only be treated by liming the field for the next crop”. While it is possible that the Ca in high application rates of gypsum may have some beneficial effect on copper contaminated soils, we believe that growers should not expect dramatic improvements, if any at all, from gypsum. Based on these findings we conclude that, if a soil contains toxic levels of Cu, applications of gypsum will have only marginal benefits at best. All known options for alleviating Cu toxicity have significant other negative effects. The best management approach is to avoid contaminating the soil with Cu in the first place.
Iron Toxicity
Direct iron toxicity is extremely rare, therefore there is little need to apply gypsum to correct a problem that does not exist, regardless of any potentially beneficial effect that gypsum may have.
Manganese Toxicity
Manganese toxicity is virtually always associated with excessively acid soils. The solution to this is to correct the soil pH with lime. Therefore, there is no place for gypsum in this situation.
Zinc Toxicity
Zinc toxicity is also extremely rare in most soils and plant production situations. Therefore, any benefit that gypsum may have on reducing Zn toxicity is a solution to a problem that generally does not exist. One exception to this is in peanut production. Peanuts are exceptionally sensitive to Zn and can develop toxic reactions at soil Zn levels much lower that other plants. However, any benefits that gypsum may have for alleviating Zn toxicity in peanuts is probably already being taken advantage of by peanut growers. Peanuts are very responsive to having abundant amounts of soluble Ca in the soil zone where the nuts are forming. Knowing this, most growers already apply gypsum to each crop to insure a quality crop. Therefore, they are unlikely to gain from higher rates of gypsum.
Calcium's Role in Reducing Ammonia Volatilization from Urea and UAN Fertilizers
Beginning in the early 1980's Dr. Lloyd Fenn, of Texas A&M University began publishing the results of his research into using soluble calcium additives to reduce ammonia volatilization from urea applied to calcareous soil. It is beyond the scope of this paper to evaluate all of the work done in this area. However, we will give a brief outline of the work.
It is well known that surface applying urea to calcareous soil often results in unacceptable N volatilization losses. Doctor Fenn found that including soluble Ca in the N application significantly reduced the amount of N lost in these conditions. Additionally, he found that there were similar, but often smaller, benefits from applications of soluble K and Mg with the urea. Doctor Fenn's work was confirmed by other researchers. In order to be effective, the additional cations (Ca, K, or Mg) that were applied had to be very easily soluble, or already in solution. Most of the work appears to have used calcium chloride as the Ca source. The ratio of the cation to amount of ammonium N was critical to success, plus. The application timing of the cations and N was important as well.
Our files on this research did not have any examples where gypsum was used as the Ca source. However, it would seem that if calcium chloride works, high quality gypsum might also work reasonably well under the same conditions and methods. The problem with recommending the practice of using co-applied cations with urea (or other urea containing fertilizers) to reduce N loss is that first you must have a significant N loss to prevent. The benefits of this practice will be limited to the estimated value of the N losses (in nutrient costs and/or yield loss) that were prevented. Conditions where there is little chance of significant N loss include the following.
- Where urea is applied to cool soils, such as winter wheat topdress
- Where the urea will be physically incorporated
- Where urea is likely to be leached into the soil with about 1/2 inch of rainfall within a short time
- Where the soil pH is somewhat acid and there is little or no crop residue or other soil cover to prevent the urea granules from good soil contact.
Of course the cost of the added cations must also be considered. Some publications suggest that to be effective, the final Ca:N ratio must be about 1:4. However, other investigators found that the required Ca:N ratio needed to be approximately 2:1 for significant effect on N loss. If a 1:4 ratio was effective in most situations, this practice might be economical. However, if the 2:1 ratio is needed, it is hard to see where the benefits will be.
Since we can't easily or accurately predict the potential volatilization losses or their effects on yield, nor have researchers been able to give us a reliable Ca:N ratio, we really can't predict the economics of this practice. It is probably instructive to note that this work has been known by agronomists since its first publication and since then few agronomists have found it valuable enough to recommend.
A Final Note
Many growers will see promotions for gypsum use that emphasize the many beneficial roles that Ca plays in both plants and soils. Most of them are probably true, to one degree or another. However, the fact that these claims may be true does not mean that a grower needs gypsum or any other Ca source. The vast majority of soils contain abundant Ca and applying more will gain nothing for the crop or the grower. Before spending scarce money on inputs based on nothing more that claims, the grower must accurately evaluate his crops needs. This requires the proper use of soil tests, plant analysis, and some time spent evaluating research results and/or on-farm field trials. Scarce input dollars must be spent where there are the greatest chances for the largest returns on investments. In most cases, the best returns will be from the proper use of more conventional, such as lime, fertilizer, seed, etc.
References
- Gypsum for Agricultural Use in Ohio-Sources and Quality of Available Products, Ohio State University Extension Fact Sheet, ANR-20-05.
- Effects of Exchangeable Ca:Mg Ratio on Soil Clay Flocculation, Infiltration and Erosion, Katrina Dontsova, L. Darrell Norton, 10th Int'l Soil Cons. Org. Meeting, 5/24-29, Purdue Univ., 1999
- Gypsum Amendment and Exchangeable Calcium and Magnesium Affecting Phosphorus and Nitrogen in Runoff, N. Favaretto, L.D. Norton, B.C.Jones, and S.M. Brouder, Soil Sci. Soc. Am. J. 70:1788-1796 (2006)
- Uses of Ground Sheetrock (Gypsum) As A Soil Amendment, Soil Science Notes, No. 1, NC State Univ. 1995
- Infiltration and Erosion in Soils Treated with Dry PAM and Gypsum, Jian Yu, et al, Soil Soc. Am. J. 67:630-636 (2003)
- Boron and Its Role in Crop Production, U.C. Gupta, CRC Press, 1993
- Trace Elements in Soils and Plants, Alina Kabata-Pendias, CRC Press, 2001
- Nutrient Management for South Carolina Based on Soil-Test Results, EC 476, Clemson University
- Plant Tissue Analysis and Interpretation for Vegetable Crops in Florida, G. Hochmuth, et al, University of Florida, Institute of Food and Agricultural Sciences, Hs964, 2004
- Handbook of Plant Nutrition, A.V. Barker and D.J. Pilbeam editors, CRC Press, 2007
- Ammonia Loss and Associated Reactions of Urea in Calcareous Soils, L. B. Fenn & S. Miyamoto, , Soil Soc. Am. J. Vol. 45, No. 3, May-June 1981.
- Increased Agricultural Benefits Through Cost Effect Utilization of Urea Fertilizer, MP-1608, Texas A&M University, 1986
- Improving the Fertilizer Efficiency of Urea ammonium Nitrate Solutions by adding Other Nutrients, G.J. Gascho, Journal of Fertilizer Issues, Vol. 3, No. 2, Pgs 62-65 April-June 1986