1087 Jamison Rd
Washington Court House, OH 43160
(800) 321-1562

This is an old revision of the document!
Magnesium (Mg) does not receive much attention in the popular press. However, an excellent article by Dr. Paul Tracy (MFA, Inc., Columbia, MO) in the November, 2001 issue of Today's Farmer reviewed Mg fertility and a potentially increasing problem in Missouri and other areas.
In his article, Dr. Tracy discussed and illustrated the point that growers in several areas of Missouri should to pay more attention to the need for Mg fertilizer. He noted that the “boot-heel”, in the Southeast part of the State was long known to have Mg problems, but that other areas now appear to be a concern. According to Dr. Tracy, a 1995 survey, conducted by the University of Missouri Soil Testing Laboratory found that only 12% of soil samples analyzed by that lab were low in Mg. However, 57% of soil samples from Southwest Missouri (not the boot-heel area) were low in Mg. Reviewing fertilizer practices; it was found that a much smaller percentage of both State and regional acreage received Mg fertilizer. His points illustrate both the regional nature of Mg needs and the typical lack of attention paid to this essential element.
Magnesium is an essential element for both plants and animals. In plants, Magnesium is the central atom in the chlorophyll molecule. This makes it essential for photosynthesis, the basis of plant life. It also plays other critical roles in plant growth. A shortage of Mg in forage can lead to grass tetany in cattle. Dr. Tracy points out that many livestock producers have the philosophy that low Mg in forage is not a concern if they use Mg feed supplements. While this may help the livestock, it does not correct the plant deficiency and perpetuates low yields of low quality forage. Uncorrected Mg shortages contribute to higher production costs and lower farm profits. Dr. Tracy is supported in this conclusion by Sumner (4) where he states “Although supplying Mg as mineral supplements is generally helpful, complete protection is only obtained when adequate bioavailable Mg in forage is consumed daily.”
Magnesium is absorbed by plants as the divalent cation Mg++. It is one of the three major nutrient cations in the soil. The others are calcium (Ca++) and potassium (K+). These three nutrients are involved in a competitive interaction with each other, as well as being affected by a fourth significant cation, hydrogen (H+). Hydrogen is significant because as the quantity of soil H+ increases, the soil becomes more acid and the soil pH decreases. Acid soil can significantly reduce plant uptake of Ca, Mg, and to a lesser extent K. The major nutrient cations occasionally have other, less significant competitors. For a short time after the application of large amounts of ammonium-N, the ammonium cation (NH4+) presents some competition for the other cations. This competition is normally very short-lived, in localized soil zones, and not significant to crop production. Where the soil pH is below 5.5, the soluble aluminum cation (Al+++) can also be found in significant amounts in the soil. Soluble Al not only provides another source of cation competition (Huang 6), but can be directly toxic to most crops.
Several situations that can lead to Mg shortages in plants are
Notice that in the previous list, the soil Ca:Mg ratio is not included. While the competition between Ca and Mg for uptake by crops has become a perennial topic of discussion in agriculture, it has not been shown to be a factor in Mg availability to plants.
Generally, the discussion about soil Ca:Mg ratio is focused on the claim by some that there is (or is not) an “ideal” soil Ca:Mg ratio that should be achieved through fertilization. It is reported that the first publication of an ideal Ca:Mg ratio came from New Jersey in 1901. This early work recommended a total Ca to total Mg ratio in the soil of about 1.25. As we know today, and was recognized soon after this publication, an analysis of the total soil content of a nutrient bears little relationship to its crop availability. Later, again in New Jersey, it was reported that the “ideal” alfalfa soil should have cation saturation's of 65% Ca, 10% Mg, 5% K, and 20% H (Haby et al 5). The later New Jersey report, plus aggressive marketing by its supporters appears to be the basis of the claims made today about an “ideal” cation balance in the soil. In the years since this claim was made, there have been numerous investigations into the possibility of an “ideal” soil cation balance. None of them have confirmed the existence of such an “ideal” balance. Fertile soils normally contain a Ca:Mg ratio between 5 and 8. However, research results show that this ratio can be as narrow as 2 or as wide as 11 without negative effects, assuming that there is an adequate amount of each nutrient in the soil.
In the mid-1980's the University of Wisconsin conducted research into the effect of Ca:Mg ratio on alfalfa growth (Shulte 7). They found that while the Ca:Mg ratio in the plant tended to reflect the soil Ca:Mg ratio, the plant content of these nutrients was affected much less than the soil ratio would suggest. In no case did the soil or plant ratio affect yield. In this work the plant Ca and Mg contents were never below the respective critical levels for each nutrient, even though the soil Ca:Mg ratios ranged from 2.28 to 8.44. They concluded that, as long as there were adequate levels of Ca and Mg present in the soil, variations in the Ca:Mg ratio over the range 2 to 8 have no effect on yield.
In 1999 the University of Missouri, Delta Research Center published the results of an investigation (Stevens 8) into the effects of soil Ca:Mg ratio on cotton. They amended plots with gypsum or Epsom salts to create soil Ca:Mg ratios between 3.8 and 11.7. They found that cotton yields were not significantly different between treatments. Additionally, soil varying Ca:Mg ratios had no significant effect on K uptake.
Other investigators have found similar results. The obvious conclusion from this body of work is that, the particular Ca:Mg ratio in the soil is rarely of any practical consequence as long as there are adequate amounts of each nutrient available to the crop.
McLean, et al (1), could find no specific cation ratios that predicted sufficiency or shortages of K, Mg, or Ca in several crops (Table 1). Notice that for all crops the Ca:Mg ratios of both the high and low yielding groups have essentially the same ranges. It doesn't appear that there is a trend or bias in the relationships between the Ca:Mg ratio and the relative yields of any crop. This indicates that the soil Ca:Mg ratio had little or no effect on yield.
| Table 1 | |||||
|---|---|---|---|---|---|
| Cation Ratio | Yield Group | Corn | Soybeans | Wheat | Alfalfa |
| Ca:Mg | High | 5.7 - 20.6 | 5.7 - 14.9 | 5.7 - 14.0 | 6.8 - 26.8 |
| Ca:Mg | Low | 5.4 - 18.8 | 2.3 - 16.1 | 6.8 - 21.5 | 5.7 - 21.5 |
| K:Mg | High | 0.33 - 1.0 | 0.33 - 1.0 | 0.32 - 0.91 | 0.4 - 1.67 |
| K:Mg | Low | 0.48 - 1.1 | 0.28 - 1.43 | 0.48 - 1.43 | 0.4 - 1.10 |
At the risk of over-analyzing this data, it appears to contain some evidence of a different relationship between K and Mg, than with Ca and either nutrient. The high yielding groups of corn and wheat (notice that they are grass) include K:Mg ratios between about 0.3 and 1.0. The low yielding groups of these crops have shifted toward a narrower and somewhat higher range (0.48 to between 1 and 1.5). This appears to suggest that relatively higher K:Mg ratio may be associated with lower yields of these grass crops. The non-grass crops (soybeans and alfalfa) have similar low-end K:Mg values in both the low and high yield groups. However, the upper end of the K:Mg range in the low yield group is much higher in soybeans than in alfalfa. This may suggest that alfalfa is more tolerant of high soil K:Mg ratios that are soybeans. These conclusions would be consistent with our experience.
While there is little evidence to support the existence of a critical soil Ca:Mg ratio, there is evidence that the soil K:Mg ratio can be important.
Rahmatullah and Baker (2) found a significant correlation between the uptake of Mg by seedling corn and the relative amounts of K and Mg in the soil.
Stout and Baker (3) found that “The differential adsorption of potassium was found to be the controlling factor in the uptake of magnesium by corn seedlings in two Pennsylvania soils.”
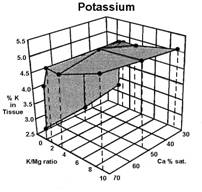
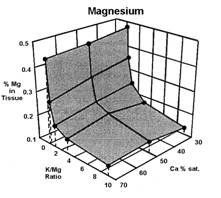
Data presented by Sumner (4) suggests a similar K:Mg relationship. In Sumners Handbook of Soil Science, work by Wilkinson et al., 1987 reported that “High rates of applied K to cool season grass pastures, whether from manure or inorganic fertilizers, increase the incidence of grass tetany”. Work by Johannsonn and Hahlin, 1977, presented by Sumner (4) illustrates the significance of the relationship between K and Mg, and the lesser effect of Ca on this 3-way interaction. In this study, the uptake of either K or Mg by oats (Figure 1, 2, and 3), was significantly decreased by increasing amounts of the other. While the availability of Ca was also important, its effect was less than either K or Mg.
| Figure 1 | Figure 2 | Figure 3 |
|---|---|---|
 |
 |
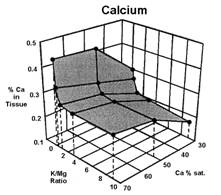 |
Several important aspects of this data are worth remembering.
Keep in mind that the crop used to generate the data in figures 1, 2, and 3 was oats, a grass. Non-grass species would be expected to have somewhat similar results, but probably not as dramatic.
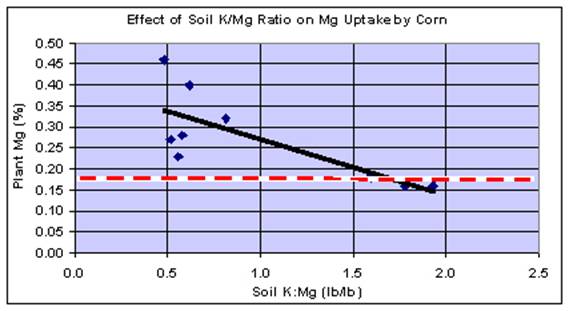
A set of customer data in the year 2000 (Figure 4) illustrates the typical situation found in corn. A fertilizer dealer sent us a group of samples taken from four of his customers farms. The soiltest levels of K and Mg, as well as the fertilizer K2O rates were somewhat different, but not dramatically so. As we looked at the results, it became apparent that there seemed to be a pattern. We looked at the data in several ways, attempting to find a formula that might explain the various Mg levels in the corn samples. The only thing that appeared to be related was the soil K:Mg ratio. Neither soil Ca levels, rates of K2O applied, nor any combination of soil Ca, Mg, K, and K2O applied improved the relationship. The important point in Figure 4 is that as the soil K:Mg ratio increases above the range of 1.0 - 1.5, the Mg level in the leaves decreased significantly. When this ratio is below 1.0, there is little, if any relationship between K and Mg on plant Mg uptake.
| Figure 4: Critical Mg level at “brown silk” |
|---|
 |
In each year since 1963, when our lab opened, we see examples of the effect of K on Mg uptake. This experience generally agrees with the examples presented here. While there is data that conflicts with the K:Mg ratio data presented here, our long experience with plant analysis leads us to conclude that
The ratio of one item to another can be expressed in several ways. For example, a ratio of 2 to 1 can be written as 2:1, 2/1, or simply as 2. In reality, a ratio is the division of the first or top number by the second or the bottom number. Therefore, if a ratio was 5 to 4, it could be expressed as 5:4, 5/4 or 1.25. The single number expression simply means that the 5 is 1.25 times as large as the 4. Ratios are most commonly expressed by the the single number method.